C1.4: Synthesis of Metal and Semiconductor Clusters in High-Boiling Solvents
Subproject Leader: Claus Feldmann
Institut für Anorganische Chemie, KIT
Contributing Scientists:
Present: Peter Leidinger
Past: Fabian Gyger
Nanocale Hollow Spheres
Nanoscale hollow spheres are intensely discussed with regard to a wide range of specific properties (e.g., large specific surface, low specific weight, container-type morphology) as well as for certain types of functionality (e.g., catalysis, gas storage, low-weight building materials, drug delivery). Subproject C1.4: Nanoscale Hollow Spheres addresses the synthesis and characterization as well as functionality and application of these nanomaterials with its specific morphology [1,2].
Microemulsion Based Synthesis
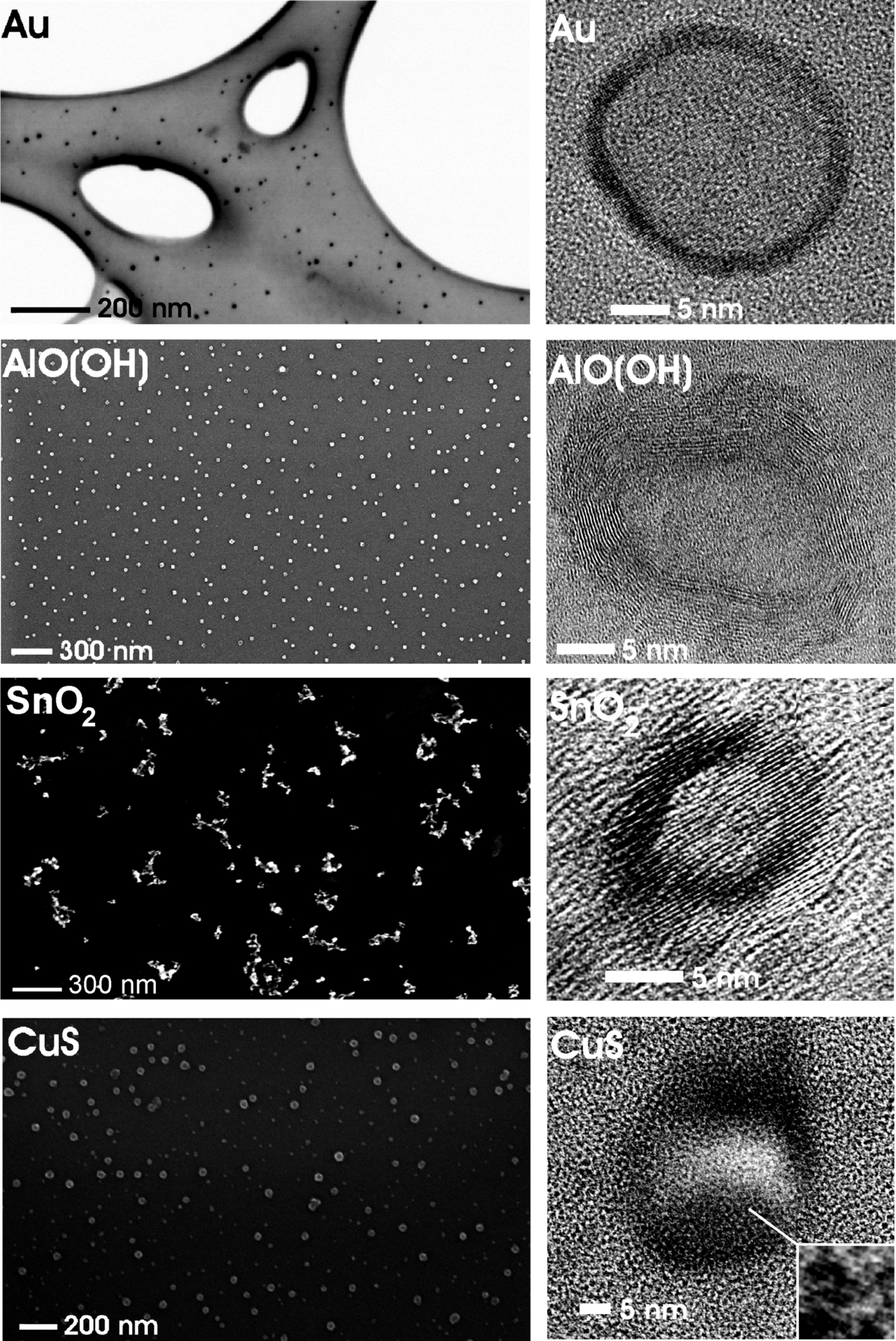
Nanoscale hollow spheres with a wide range of compositions are here realized via a newly developed microemulsion-based approach. This includes oxides (e.g., g-AlO(OH), La(OH)3, ZnO, SnO2, TiO2), sulfides (e.g., CuS, Cu1.8S, Cu2S, Ag2S) and metals (e.g., Au, Ag) [2-5]. The hollow spheres exhibit outer diameters of 10–50 nm, a wall thickness of 2–10 nm and an inner cavity ranging from 5 to 30 nm in diameter. Outer diameter and inner cavity size of the hollow spheres can be selectively adjusted during synthesis via the size of the initial micelles [5].
Use as Sensor, Catalyst, Nanocontainer as well as Gas Sorption/Separation
With specific surfaces of up to 600 m2g–1, nanoscale hollow spheres (e.g. SnO2, Fe2O3, TiO2) become highly relevant for sensors as well as for catalysis [1,2]. The possibility to selectively modify the inner or outer surface of the hollow spheres gives access to an additional degree of freedom. In view of the inner cavity, hollow spheres can be furthermore used as a nanocontainer to encapsulate inorganic salts (e.g., KF, KSCN, K2S2O8), biomolecules (e.g., phenylalanine, quercetine, nicotinic acid) or fluorescent dyes (e.g., rhodamine, riboflavine) [2–5]. And finally, gas sorption and gas separation come into range based on remarkable CO2 uptakes of about 200–300 mg g-1.
References
|
[1] |
H. Goesmann, C. Feldmann, Nanoparticulate Functional Materials, Angew. Chem. Int. Ed. 2010, 49, 1362 (Review) |
|
[2] |
H. Gröger, C. Kind, P. Leidinger, M. Roming, C. Feldmann, Nanoscale Hollow Spheres: Microemulsion-based Synthesis, Structural Characterization and Container-type Functionalities, Materials 2010, 3, 4355 (Review) |
|
[3] |
D. H. M. Buchold, C. Feldmann, Nanoscale AlO(OH) Hollow Spheres - Synthesis and Container-type Functionality, Nano Lett. 2007, 7, 3489 |
|
[4] |
H. Gröger, F. Gyger, P. Leidinger, C. Zurmühl, C. Feldmann, Microemulsion Approach to Nanocontainers and its Variability in Composition and Load, Adv. Mater. 2009, 21, 1586 |
|
[5] |
P. Leidinger, R. Popescu, D. Gerthsen, C. Feldmann,Nanoscale La(OH)3 Hollow Spheres and Fine-tuning of Its Outer Diameter and Cavity Size, Small 2010, 6, 1886 |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF