F2.1: Nanostructured Functional Layers for Advanced Oxygen Separation Membranes
Subproject Leader:
| Dagmar Gerthsen | Laboratorium für Elektronenmikroskopie, KIT |
| Ellen Ivers-Tiffée | Institut für Werkstoffe der Elektrotechnik, KIT |
Contributing Scientists: Levin Dieterle, Philipp Müller, Heike Stömer, Mónica Burriel, Christian Niedrig, Stefan Wagner
The Reduction of CO2 Emissions
Electrical power plants fueled by coal or gas produce CO2 emissions which have to be reduced by 50 to 60 % by 2050, as mandated by the European Commission. This major issue can be addressed, e.g., by oxyfuel combustion (burning a fuel in pure oxygen which results in a CO2-rich flue gas) and subsequent sequestration of the CO2 produced by established carbon capture and storage (CCS) techniques.
For oxyfuel combustion, focus is on the generation of pure oxygen from air by means of oxygen separation techniques. This can be achieved by making use of membrane technology, an approach considered energetically favourable compared to cryogenic oxygen production.
Oxygen-Separation Membranes
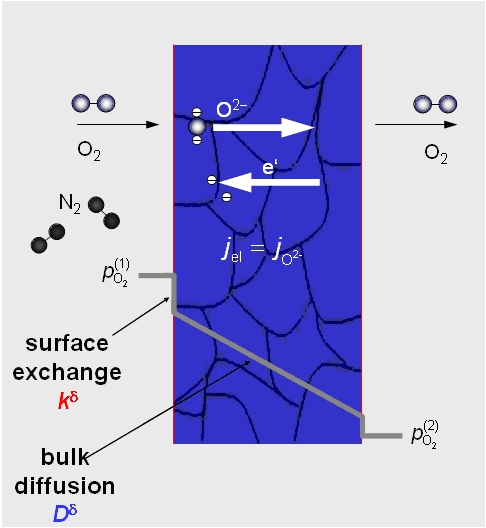
Oxygen can be produced by using a dense ceramic membrane that separates two gas atmospheres with different oxygen partial pressures. At high temperatures (e.g., 800 °C) oxygen molecules from pressurized air dissociate at the outer membrane surface and are transported through the solid oxide as oxygen ions to the inner side where they leave the membrane and form oxygen molecules again. In this way, pure oxygen can be separated “from thin air”.
As the transport of oxygen ions involves ionic charge transport through the membrane, this has to be met by a counter-transport of electrons. This can be best achieved by using mixed ionic-electronic conducting (MIEC) materials. Among these, the perovskite Ba0.5Sr0.5Co0.8Fe0.2O3-δ (BSCF) in its cubic phase shows outstanding oxygen permeability and selectivity for oxygen/air separation.
Where does “Nano” Come Into Play?
The oxygen flux through the membrane can be increased by decreasing the membrane thickness. However, upon reducing the thickness below a certain “characteristic” membrane thickness (given by the ratio of oxygen diffusion constant Dd and oxygen surface exchange coefficient kd for the material employed), surface reactions become rate-determining. Therefore, no increase in the oxygen flux is achieved by further decreasing the membrane thickness since this limit is reached [1].
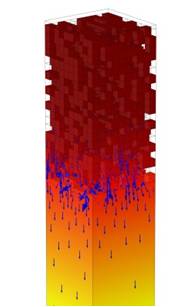
Hence, our main target is to boost oxygen surface exchange by nanostructuring the gas/membrane interface. This yields a tremendous enlargement of the electrochemically active surface area available for oxygen reduction and, thus, significantly enhances the oxygen flux.
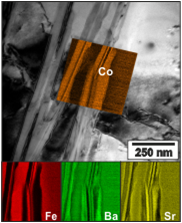
Phase Stability of BSCF
By a combination of electrochemical high-temperature measurements in Ellen Ivers-Tiffée’s group and highly sophisticated TEM investigations in Dagmar Gerthsen’s group it was possible to validate that cubic BSCF is, however, not stable in the temperature range around 800 °C. The formation of further (unfavourable) crystalline BSCF phases on a very long time scale [2], and thereby the reduction of electrochemical performance was clearly demonstrated [3-4]. Hence, enhancement of structural stability of cubic BSCF material under application-relevant conditions (high temperature, low oxygen partial pressure) is a prudent goal. A promising route could be co-doping on the B-lattice site.
Nanoscaled Functional Layers
In collaboration with partners from the University Barcelona and Dirk Fuchs (KIT-IFP), we have succeeded to deposit high-quality epitaxial BSCF nanoscale thin films by pulsed-laser deposition. This facilitated the first-time evaluation of intrinsic electrical conductivity as well as oxygen surface exchange properties in single-crystalline samples.
In a next step, nanoporous functional layers deposited onto BSCF and their influence on oxygen surface exchange are going to be studied. These layers are either made of the membrane material (resulting in a vastly increased electrochemically active surface area), or of another material that shows catalytical activity for the promotion of oxygen transport. The resulting layers will be analyzed electrochemically and by high-resolution transmission-electron microscopy, also including analytical electron microscopy.
References
|
[1] |
M. Burriel, C. Niedrig, W. Menesklou, S. F. Wagner, J. Santiso, E. Ivers-Tiffée, Solid State Ionics 181, 602 (2010) |
|
[2] |
P. Müller, L. Dieterle, E. Müller, H. Störmer, D. Gerthsen, C. Niedrig, S. Taufall, S. F. Wagner, E. Ivers-Tiffée, ECS Transactions 28, 309 (2010) |
|
[3] |
C. Niedrig, S. Taufall, M. Burriel, W. Menesklou, S. F. Wagner, S. Baumann, E. Ivers-Tiffée, accepted for publication in Solid State Ionics (2011) |
|
[4] |
P. Müller, H. Störmer, L. Dieterle, C. Niedrig, E. Ivers-Tiffée, D. Gerthsen, submitted to Solid State Ionics (2011) |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF