C4.9: Electrochemistry with an Electron Beam – Local Metal Deposition in Ionic-Liquid and Molten-Salt Thin Films
Subproject Leader: Rolf Schuster
Contributing Scientists:
Present: Vadym Halka
Past: Matthias Schmid, Vasevolod Avrutskiy
Drawing with an Electron Beam
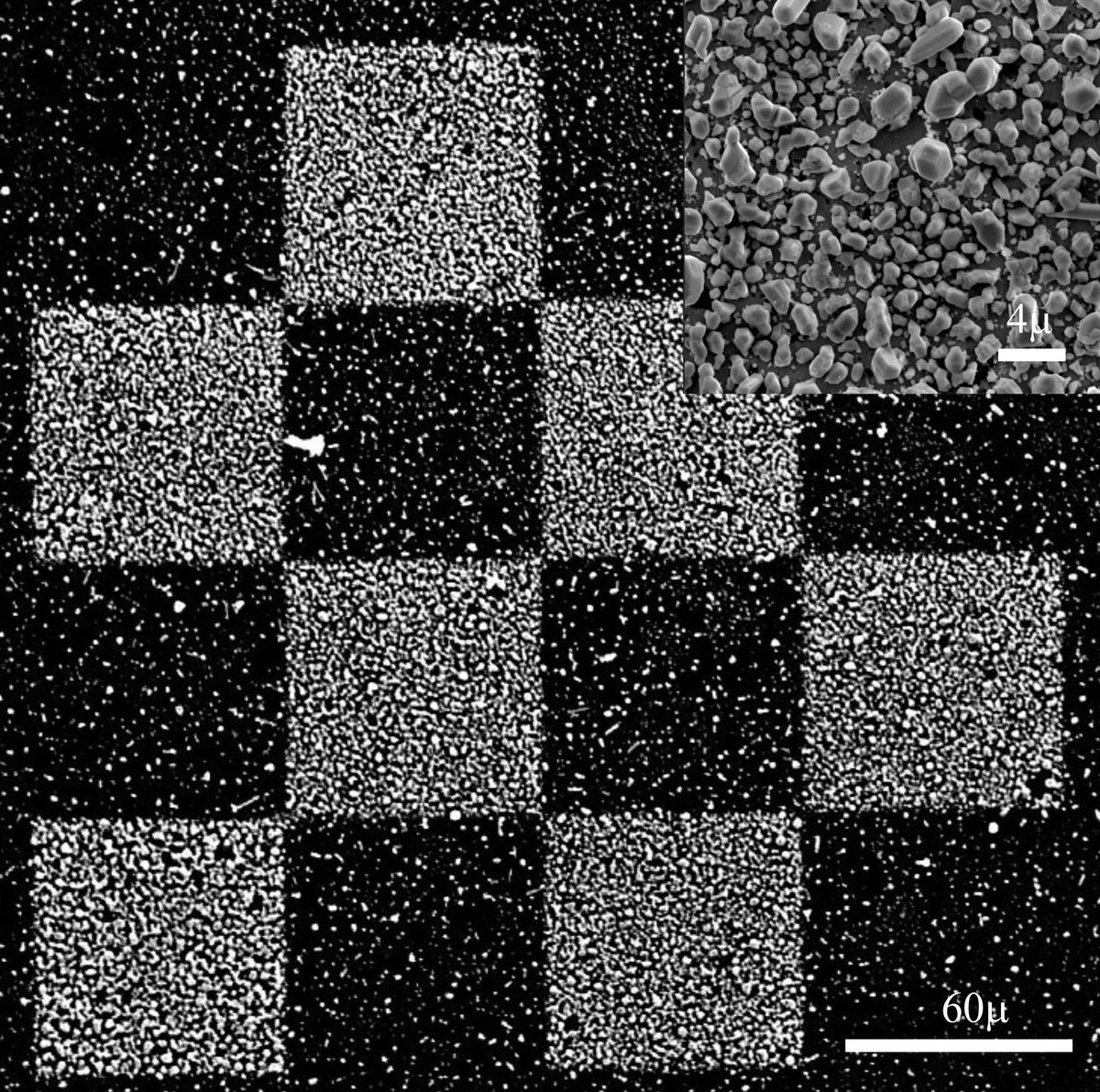
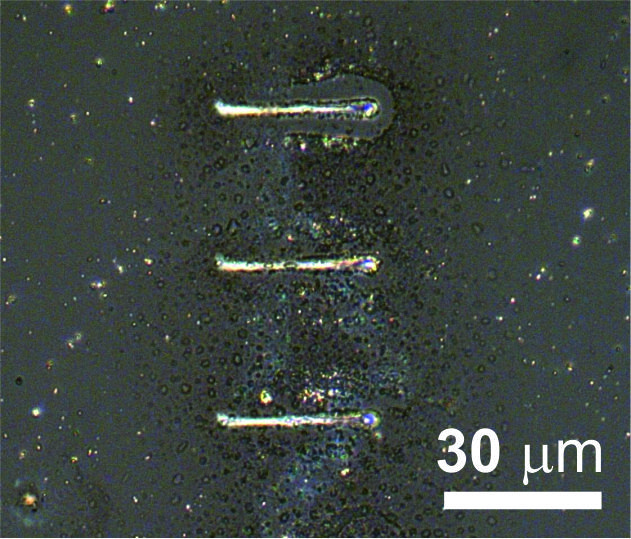
In subproject C4.9: "Electrochemistry with an electron beam – local metal deposition in ionic-liquid and molten-salt thin films" the electron beam of an SEM was used to fabricate metallic microstructures from molten salt films. The objective of the project is to tune the morphology of the deposits from isolated nanoparticles to compact metal deposits by variation of the irradiation parameters.
Motivation
The idea of metal particle generation from an electrolyte using free electrons is not new. In 1877 J. Gubkin [1] performed experiment with aqueous silver nitrate solutions, where he reduced the Ag ions by free electrons from a glow discharge, which led to the formation of Ag nanoparticles in the solution. Recently J. Janek, F. Endres and their coworkers [2] used ionic liquids (IL) as solvent for silver salts, which, due to the low vapor pressure of the IL, allowed better control of the glow-discharge. Here we use a focused electron beam for the local metal deposition from micrometer-thin electrolyte films.
Results
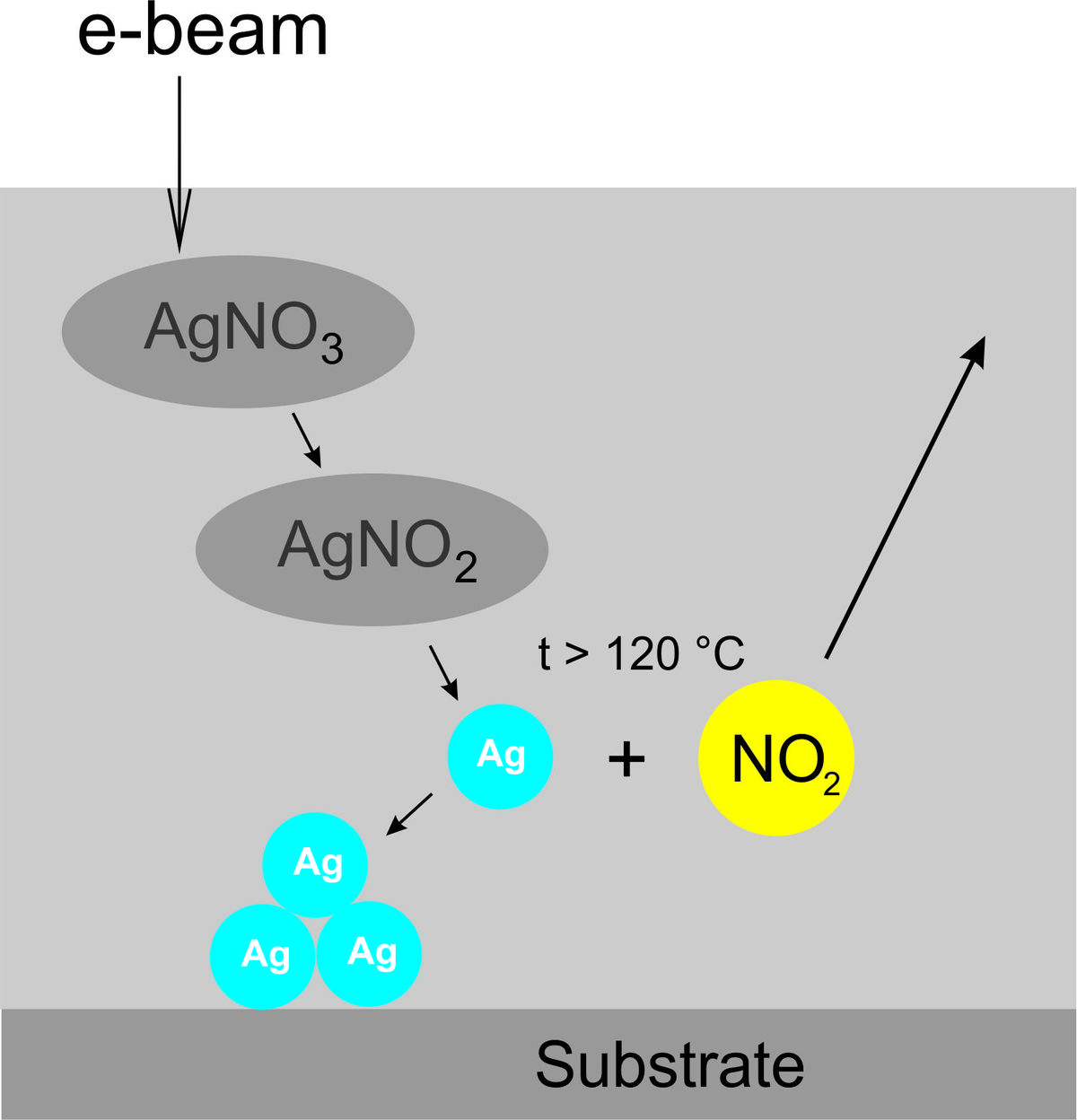
Due to the electric conductivity of the molten salt film, in the present case AgNO3, metallic patterns can be written onto conductive, semiconductive and nonconductive substrates [3]. The strong dependence of the morphology of deposits on the irradiation parameters reflects the importance of nucleation and growth processes. Because of the good wetting capability of the electrolyte film, thick, compact Ag structures can be grown. In the case of silver deposition from molten silver nitrate the reaction proceeds in three steps, i.e., (i) reduction of NO3 to NO2 by the incident e-beam, (ii) thermal decomposition of the formed AgNO2 into metallic Ag and NO2, and (iii) precipitation of Ag atoms/clusters from the electrolyte onto the surface.
References
|
[1] |
J. Gubkin, Ann. Phys. Chem. N. F. 32, 114 (1887) |
|
[2] |
J. Janek, F. Endres et al., ChemPhysChem 8, 50 (2007) |
|
[3] |
V. Halka, M.J. Schmid, V. Avrutskiy, X. Ma, R. Schuster, Angewandte Chemie 50, 4692 (2011) |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF