C1.3: Synthesis and Structural Characterization of Metalloid Al and Ga Clusters
Subproject Leader: Hansgeorg Schnöckel
Institut für Anorganische Chemie, KIT
Contributing Scientists:
Present: Mateusz Zauliczny, Tomasz Kruczynski
Past: Patrick Henke, Florian Henke, Christian Schenk, Lukasz Ponikiewski
Structures and properties of metalloid Al and Ga clusters open our eyes for the diversity and complexity of fundamental chemical and physical processes during formation and dissolution of metals.
What is the Excitement about Metalloid Clusters?
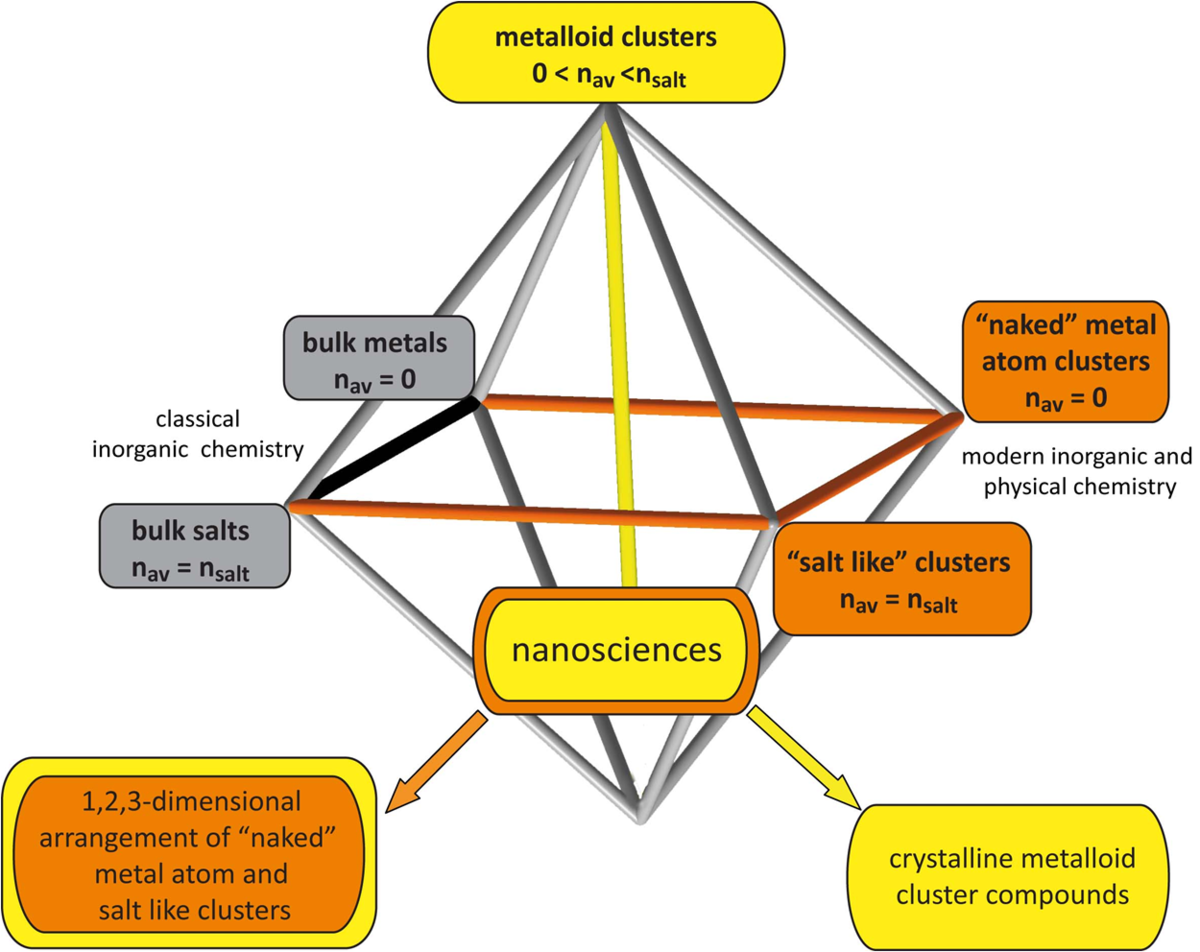
We have described clusters, which contain both ligand-bearing and naked metal atoms that are bonded only to other metal atoms, as metalloid. Thus, there are three different types of metal atom clusters: The naked metal atom clusters which are present under ultra high vacuum conditions, the metalloid clusters, and finally the giant “salt-like” clusters. The metalloid clusters represent the most complex type of cluster, as a highly mixed valence situation exists for the metal atoms, resulting in an average oxidation number between zero and the oxidation number of the salts. This falls between the much “simpler” situations of naked metal atom clusters (oxidation number 0) and the “salt-like” metal atom clusters (oxidation number nsalt). However, it should be mentioned that, because of the sophisticated methods needed for the preparation of metalloid clusters, the great majority of published results on nano-sized metal atom clusters are based on investigations with either naked metal atom clusters or salt-like clusters. Figure 1 visualizes the relation between these three types of clusters and presents a correlation with classical inorganic chemistry of the bulk phases of the metals and their salts [1].
Preparative Challenges
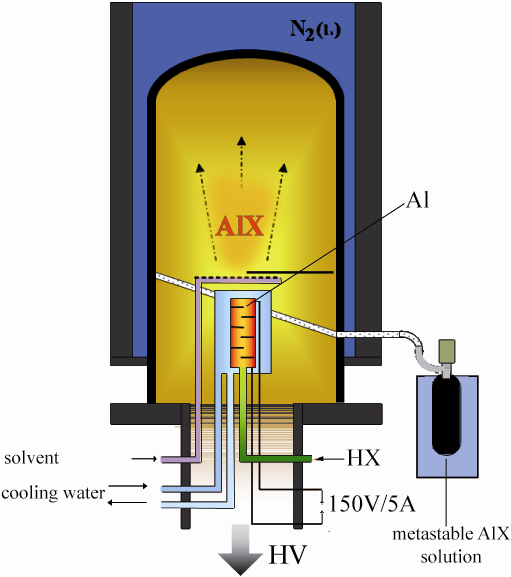
Metalloid clusters are intermediates on the way from, for example, metastable AlCl molecules to solid Al:
3 AlCl(g) → 2 Al(s) + AlCl3(g)
However, trapping AlCl high-temperature molecules at –196 °C and the generation of a metastable AlCl solution (~ -80°C) requires a high sophisticated technique which is schematically visualized in Fig. 2.
A stream of HCl gas passes the heated Al metal at 1000°C:
In order to prevent the aggregation and disproportionation of the condensed AlCl species an excess of a suitable solvent must be deposited with the monohalide molecule (e.g. toluene and a donor component) (Fig. 2).
Outstanding Results and Support via Other CFN Efforts
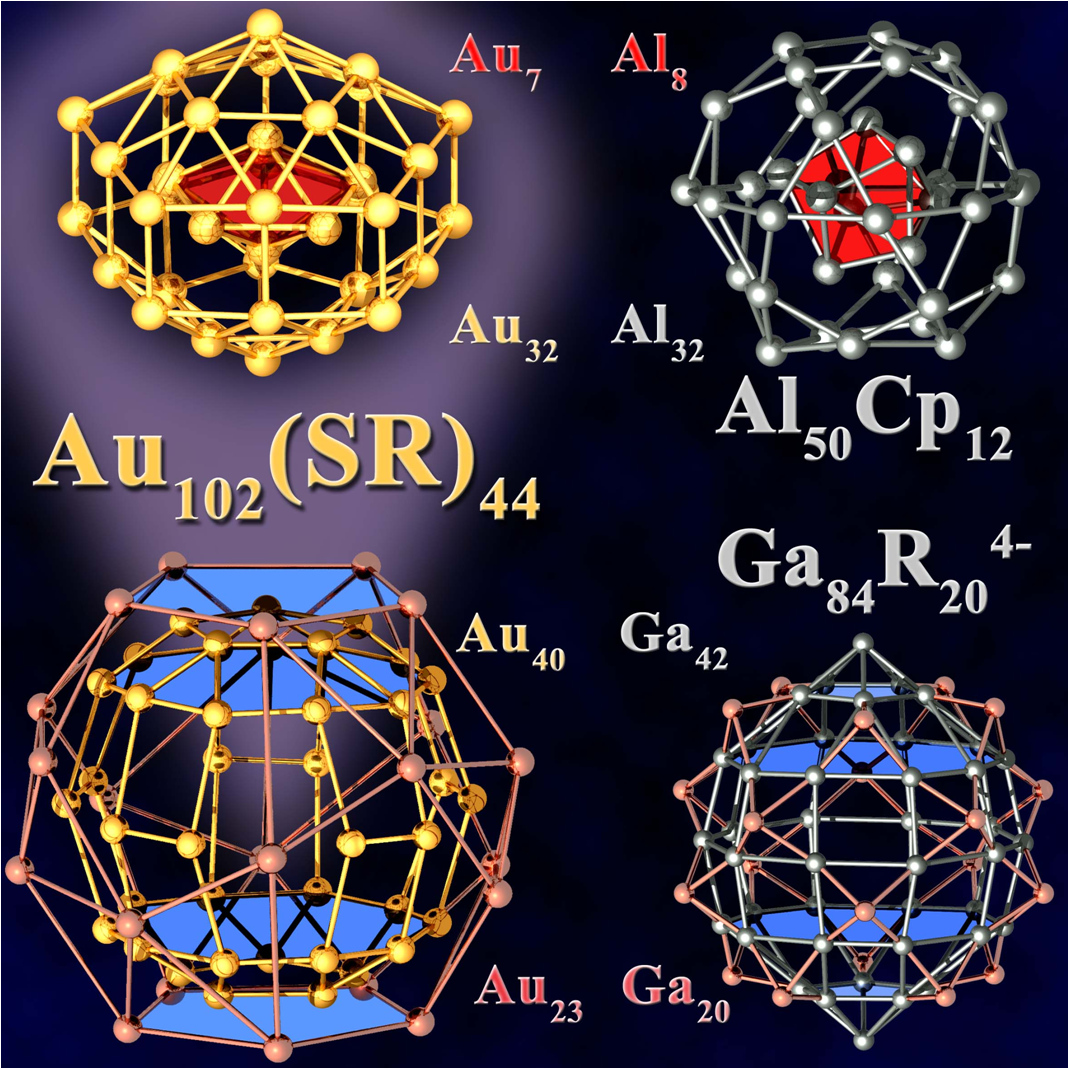
In view of the many synthetic results from metalloid clusters of precious metals (Au, Pd) with few detailed structure determinations on one hand and the failure to synthesize clusters of base metals before the last decade of the last century it came as a great surprise when in 1997 an Al77R20– cluster anion was discovered, containing 57 “naked” and only 20 ligand bearing Al atoms. From this beginning an exciting story has developed with other larger Al and Ga clusters (e.g. Al50R12, Si@Al56R12 and Ga84R20). Finally today metalloid clusters of Al and Ga exhibiting unexpected properties represent by far the largest group for this type of clusters in the whole periodic system. The relation of these clusters to a recently highlighted Au102 cluster has been discussed recently [2].
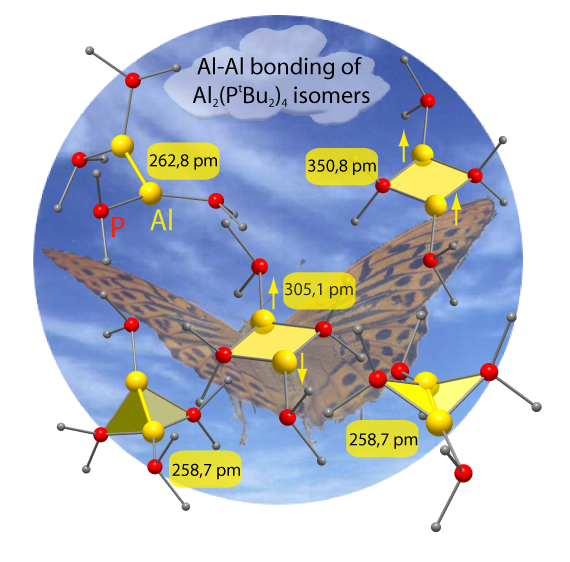
In order to understand primary steps of the formation of e.g. AlAl bonds, our synthetic results have been supported by theoretical chemistry of the CFN [3].
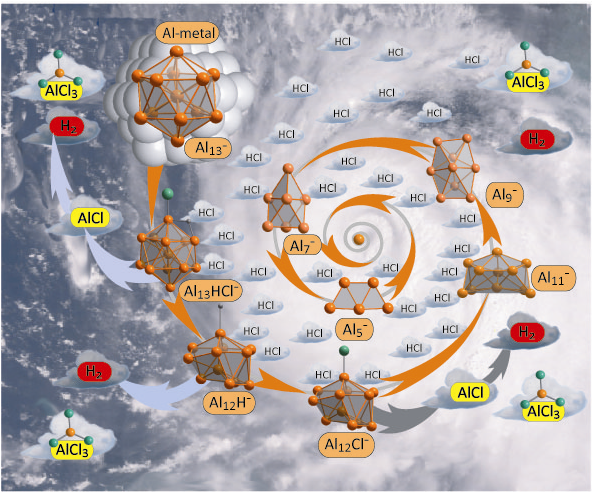
Furthermore, the understanding of the dissolution process of metals via primary steps (an Al13– cluster, as a model for the Al bulk, reacts step by step in the gas phase with gases like HCl, Cl2 and O2) has been supported by other groups of the CFN family with FT ICR mass spectrometry [4].
Future Work
After the successful formation of the first Mg(I) halide high-temperature molecule and its spectroscopic characterization recently [5] we just have upscaled the generation to a synthesis style. Therefore, to advance the field of metalloid magnesium clusters and thus create a new potential for magnesium-rich compounds, the synthesis of non-chelate-stabilized reaction species (e.g. Mg2Cp*2) is an absolute prerequisite. Investigations on the generation and crystallization of Mg-rich species like e.g. MgnCp*m (n > m) have been proved to be extremely unstable (e.g. the formation of Mg-metal, Fig. 6, is thermodynamically favored) during first experiments, which are focus of our current research.
References
|
[1] |
H. Schnöckel, Chem. Rev. 110, 4125 (2010) |
|
[2] |
A. Schnepf, C. Schenk, P. Henke, and H. Schnöckel, Z. Anorg. Allg. Chem. 637, 15 (2011); DOI: 10.1002/zaac201000333 |
|
[3] |
P. Henke, T. Pankewitz, W. Klopper, F. Breher, and H. Schnöckel, Angew. Chem. Int. Ed. 48, 8141-8145 (2009) |
|
[4] |
R. Burgert and H. Schnöckel, Chem. Comm. Feature Article 18, 2075 (2008) |
|
[5] |
R. Köppe, P. Henke, and H. Schnöckel, Angew. Chem. 120, 8868 (2008); Angew. Chem. Int. Ed. 47, 8740 (2008) |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF