E2.4: Cell Adhesion and Migration on Micro- and Nanostructured Substrates
Subproject Leader: Clemens Franz
Young Scientist Group - CFN, KIT
Contributing Scientists:
Present: Anna Burcza, Lu Dao, Tetyana Gudzenko, Dimitar Stamov
Past: Anna Müller, Jochen Dindorf
Young Scientist Group Nanobiology at the CFN
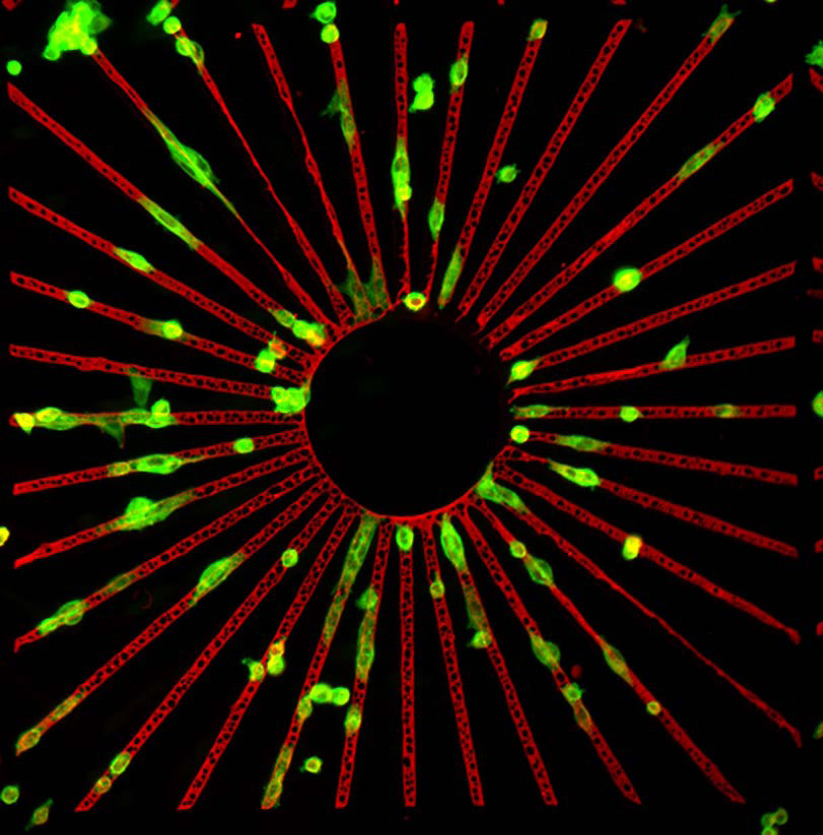
We are interested in better understanding fundamental mechanisms of cell adhesion, particularly in the initial adhesion events when cells first encounter components of the extracellular matrix (ECM). Through the use of micro- or nanopatterned artificial cell adhesion substrates, we also aim to emulate the natural cell environment and to manipulate cell adhesion directly.
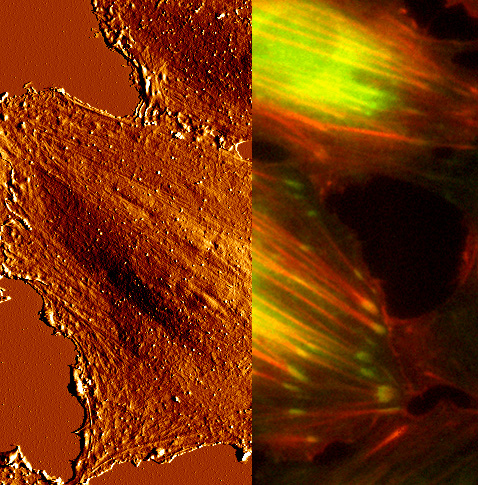
Imaging Biological Samples by AFM
In our work we frequently atomic force microscopy (AFM) and we are interested in expanding the use of AFM for cell biological applications. The AFM, a member of the scanning probe microscopy family, operates by moving a nanoscopically sharp tip of the sample surface. Samples can be imaged directly without prior preparation, such as staining or fixation. This makes AFM extremely suitable for imaging biological samples, since biological molecules or even living cells can be maintained under physiological conditions. We routinely use AFM to characterize cell adhesion substrates.
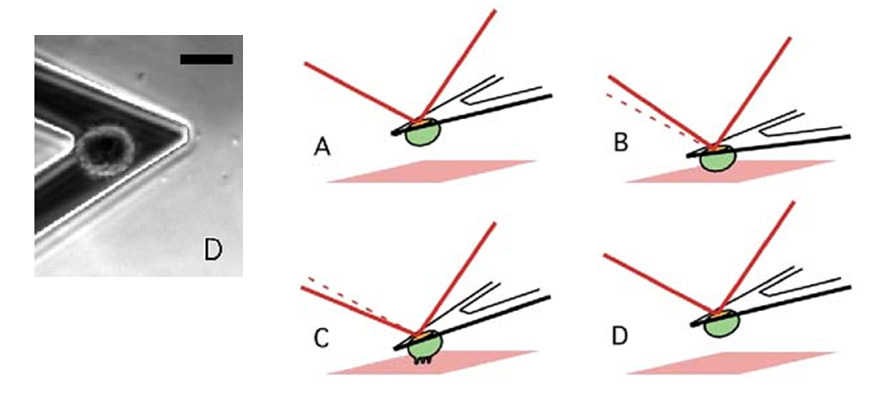
Measuring Cellular Forces
AFM cantilevers are ultrasoft springs and can be used to measure inter- and even intramolecular bonds. To study cell adhesion, we often employ AFM-based single-cell force spectroscopy (SCFS). In SCFS, a living cell is attached to the AFM cantilever and brought into contact with a substrate under defined contact conditions (contact force and time). With a force sensitivity spanning over four orders of magnitude, SCFS provides a unique opportunity to measure cellular adhesion forces from the single-molecule level to overall adhesion in the same experimental setup. AFM-based SCFS has provided important insight into molecular mechanisms involved in adhesion force generation, such as the transition from single-receptor mediated to cooperative receptor binding during the initial phase of cellular contact with an extracellular substrate. Furthermore, the adhesive properties of different surfaces can be accurately characterized.
Integrating AFM and Light Microscopy
A current challenge is to combine AFM with advanced light microscopy techniques, such as laser scanning confocal or total internal reflection (TIRF) microscopy. In this way, the clustering of receptors can be followed in living cells by optical time-lapse-microscopy and directly correlated to the adhesion force information. Our current experimental setup includes a functional TIRF/AFM platform.
|
[1] |
F. Klein, T. Striebel, J. Fischer, Z. Jiang, C.M. Franz, G. von Freymann, M. Wegener and M. Bastmeyer, Elastic fully three-dimensional microstructure scaffolds for cell force measurements, Adv Mater 22, 868-71 (2010) |
|
[2] |
S. Engin, V. Trouillet, C.M. Franz, A. Welle, M. Bruns and D. Wedlich, Benzylguanine thiol self-assembled monolayers for the immobilization of SNAP-tag proteins on microcontact-printed surface structures, Langmuir 26, 6097-101 (2010) |
|
[3] |
C.M. Franz and P.H. Puech, Atomic force microscopy - a versatile tool for studying cell morphology, adhesion and mechanics, Cell Mol Bioeng 2008, 289-300 (2008) |
|
[4] |
C.M. Franz, A. Taubenberger, P.H. Puech and D.J. Muller, Studying integrin-mediated cell adhesion at the single-molecule level using AFM force spectroscopy, Sci STKE 2007, pl5 (2007) |
|
[5] |
A. Taubenberger, D.A. Cisneros, J. Friedrichs, P.H. Puech, D.J. Muller and C.M. Franz, Revealing early steps of alpha2beta1 integrin-mediated adhesion to collagen type I by using single-cell force spectroscopy, Mol Biol Cell 18, 1634-44 (2007) |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF