F3.1: Nanostructured Transition Metal Chalcogenides as Materials in Lithium Ion Batteries
Subproject Leader: Dieter Fenske, Andreas Eichhöfer
Institute of Nanotechnology ![]() , KIT
, KIT
Contributing Scientists: Vasyl Andrushko, Silvio Indris, Sharali Malik, Heino Sommer
Motivation: Metal Sulfides as Materials in Lithium Ion Batteries
After transition metal sulfides were initially successfully used as lithium primary cell materials, Whittingham was the first to demonstrate fast, reversible Li insertion into TiS2 over the solid-solution range 0 ≤ x ≤ 1 of LixTiS2.[1] On the other hand the technical feasibility of the electrochemical reaction of alkali metals with sulfur is also well-known and high temperature sodium-sulfur batteries are currently in use for certain stationary storage applications. In turn a battery based on lithium/elemental sulfur redox couples has a theoretical specific capacity of 1675 mAh g–1 of active material and a theoretical specific energy of 2500 Wh kg–1 assuming complete reaction to form Li2S. Unfortunately the formation of a variety of lithium polysulfide intermediates which are partially soluble in the electrolyte result in severe inefficiencies. Nonetheless, in view of the reactivity of sulfur with lithium, it is not surprising that several transition metal sulfides have been reported to react with lithium following a conversion reaction to produce Li2S and metal nanoparticles. These sulfides therefore share characteristics and issues as electrodes with elemental sulfur.
State of the Art: Copper Sulfides as Materials in Lithium Ion Batteries
It is known that CuS is a promising cathode material for use in low voltage battery systems, due to its high specific capacity of 561 mAh g–1 and good electronic conductivity of 1 x 10–3 Scm–1. Its overall conversion reaction to Cu and Li2S proceeds through an intermediate step that includes the formation of LiyCuS which has been shown to be reversible.
| CuS + Li+ + e– | ⟶ | LiyCuS (y ≈ 1) | (first process) | |
| LiCuS + Li+ + e– | ⟶ | Li2S + Cu | (second process) |
Despite the reversibility of the reaction in the first cycle, severe capacity losses are observed due to irreversible sulfur dissolution in the electrolyte upon cycling.
Own Results: The electrochemical behaviour of nanostructured lithiated copper(I)sulfide phases[5]
Two different approaches were used to synthesize lithiated copper(I)sulfide with the formal composition Li2Cu4S3. In the first approach thiolato bridged copper cluster complexes Li (dme)3][Cu4(SPh)6] and [Li(diglyme)2][Cu4(SPh)6] were decomposed/thermolized under vacuum conditions. In another approach solid state reactions of Li2S and two Cu2S were performed in quartz ampoules sealed under vacuum at 400 °C.
The as synthesized powders reveal complex room temperature XRD powder patterns. It is obvious that the patterns do not consist of simple mixtures of the binary sulfides Li2S and Cu2S. Interestingly structures of any `LiCuS´ phases have not been reported in literature to date.
SEM measurements show that the `Li2Cu4S3´ material derived from the cluster molecules is highly porous while that obtained from solid state reactions of Li2S and Cu2S is rather compact.
The electrochemical behaviour of the materials investigated in solid state in Swagelock cells shows a reversible exchange of 2 Li+ ions between 1.9 and 2.7V.
| –2Li+ | |||
| Li2CuI4S3 |
⇄ |
CuI2CuII2S3 | 147 Ah kg–1 (theo.) |
| +2Li+ |
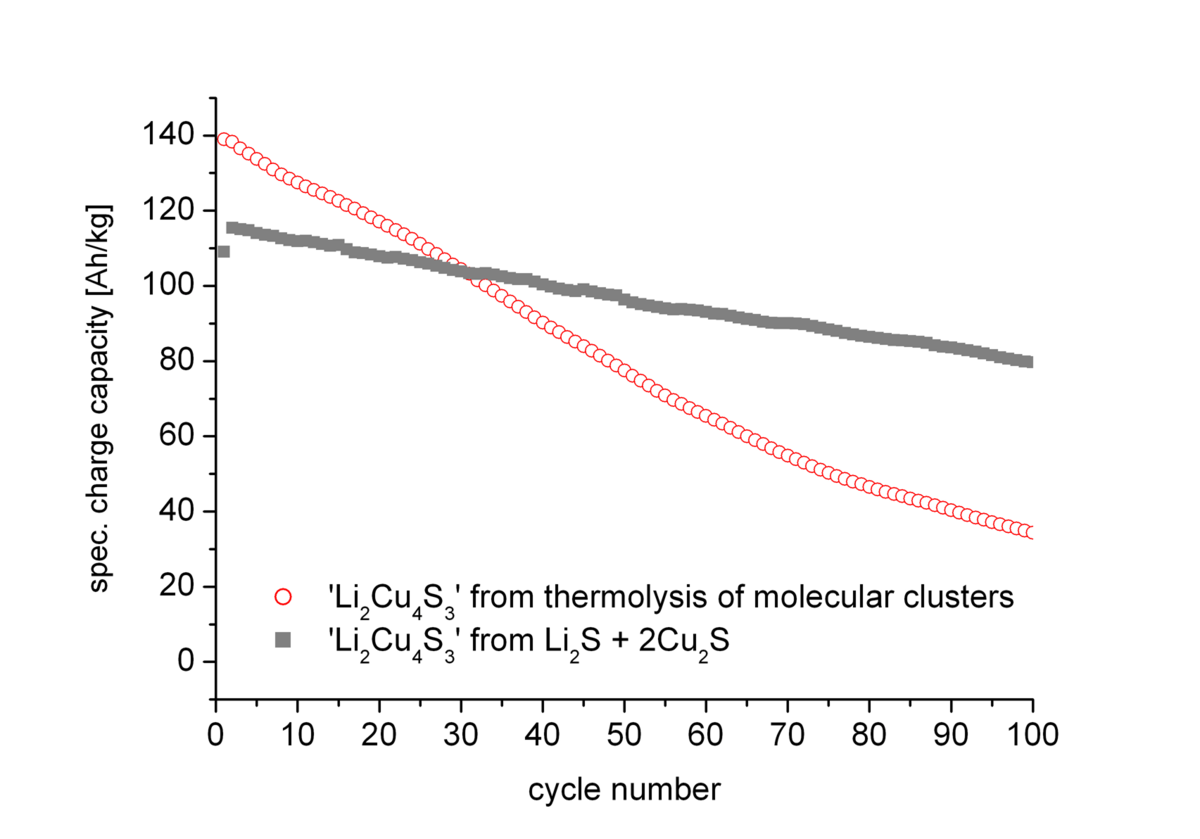
Further discharge below 1.9 V leads to a fast degradation of the material. The two materials however show significant differences in their cycling stability for the reversible exchange of the two Li+ ions (Fig. 1). While for the porous material a higher initial capacity (140 Ah/kg) than for the compact material (120 Ah/kg) was observed the former material suffers from much faster degradation during cycling.
Future investigations aim on the one hand on the synthesis of nanostructured carbon stabilized ‘LiCuS’ materials in order to improve the cycling stability and on the other hand on the synthesis of lithium rich copper sulfides e. g. `LixCu2S1+0.5x´ ( x = 4, 6) and their electrochemical characterization.
References
|
[1] |
M. S. Whittingham, Science, 1976, 192, 1126 |
|
[2] |
H. Sommer, N. Drebov, A. Eichhöfer, R. Ahlrichs, D. Fenske, Syntheses, structures and theoretical investigations of [Li(thf)4]2[Ti2Cu8S4(SPh)10] and [Ti2Ag6S6Cl2(PPhiPr2)6], Eur. J. Inorg. Chem. 2009, 28, 4329 – 4334 |
|
[3] |
A. Eichhöfer, J. Jiang, H. Sommer, F. Weigend, O. Fuhr, D. Fenske, C.-Y. Su, G. Buth, 1-D-Tin(II) Phenylchalcogenolato Complexes [Sn(EPh)2] (E = S, Se, Te) ¾ Synthesis, Structures, Quantum Chemical Studies and Thermal Behaviour, Eur. J. Inorg. Chem. 2010, 3, 410 – 418 |
|
[4] |
V. Andrushko, H. Sommer, D. Himmel, D. Fenske, A. Eichhöfer, Syntheses, structures and properties of trinuclear copper(I)/titanium(IV)-thiolate complexes, Eur. J. Inorg. Chem. 2011, 3102-3110 |
|
[5] |
A. Eichhöfer, H. Sommer, V. Andrushko, D. Fenske, S. Becker, S. Indris, S. Malik, Synthesis and Electrochemical Behaviour of Lithiated Copper(I)sulfide, manuscript in preparation |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF