F3.3: Nanocrystalline Composites Containing SnO2 as New Anode Materials for Li-Ion Batteries
Principal Investigator: Horst Hahn, Sylvio Indris, Anne S. Ulrich
Institute of Nanotechnology, KIT
Institute of Organic Chemistry , KIT
Institute of Biological Interfaces (IBG-2) , KIT
Contributing Scientists: Sebastian M. Becker, Marco Scheuermann
Introduction
The development of mobile electronic devices (notebooks, mobile phones, etc.) is strongly related to the improvement of lithium-ion batteries. The electrode materials that are capable of inserting lithium have to be optimized in order to achieve the required parameters for applications.. The use of nanocrystalline materials allows for a faster lithium insertion/extrusion due to the shorter diffusion pathways, resulting in a higher power density. SnO2 is a promising candidate for the use as an anode material in Li ion batteries since it has a high theoretical specific charge capacity. According to the subsequent reactions
4 Li + SnO2 → 2 Li2O + Sn , 4.4 Li + Sn → Li4.4Sn
a total of 8.4 Li ions can be inserted per formula unit SnO2. This corresponds to a theoretical capacity of 1491 mAh/g. While the second step is fully reversible, the first step will show only partial reversibility.
Close cooperations exist with projects F3.1 and F3.2.
SnO2 Nanostructures
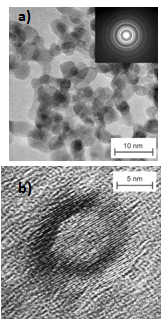
We studied the local structural disorder and relaxation in different nanostructures of SnO2 by using 119Sn MAS NMR measurements in combination with 119Sn Mössbauer spectroscopy. We investigated nanocrystalline powders with an average crystallite size of 8 nm as well as hollow spheres with a wall thickness of 3 nm and a diameter of 14 nm, and compared the results to coarse-grained materials. While the uniform SnO6 octahedra in the coarse-grained material show a well-known distortion and thus large electric field gradients, the nanocrystalline SnO2 exhibits a structural relaxation leading to a distribution of local environments and more symmetric octahedra. The SnO2 hollow spheres show strong local disorder in combination with highly asymmetric environments around the Sn atoms.
Mechanochemically prepared ZnO:SnO2 composites and Zn2SnO4
This sub-project consists of two main parts. In part one the first complete synthesis of Zn2SnO4 in a mechanochemical process is presented. Part two reports about the electrochemical characterization of this material used as electrode material in lithium-ion batteries. This characterization includes the techniques of galvanostatic cycling, cyclovoltammetry, XRD, NMR, Mössbauer spectroscopy, and in-situ SEM. Zn2SnO4 is an important material in fields like photoelectrochemical cells, photocatalysts and sensor applications. So it is in general desirable to have a simple, fast and cheap way with high yield to synthesize this material. These requirements are fulfilled by the process we used, namely the mechanochemical synthesis. Furthermore, this method operates at relatively low temperature compared to other chemical synthesis routes (Zn2SnO4 already has been synthesized by e.g. hydrothermal and solid-state reactions).
In our work nanocrystalline Zn2SnO4 powders have been prepared by a one-step mechanochemical synthesis. Using the binary precursors SnO2 and ZnO, together with adequate starting and milling conditions it was possible to synthesize a phase-pure material. The milling was carried out in a planetary ball-mill. For comparison Zn2SnO4 was also synthesized in the classical solid-state route. Here a preparation by homogenization and compaction of the reactants is needed as well as a final high-temperature treatment.
SnO2/C Nanocomposites
Nanocomposites based on SnO2 with carbon scaffold were used as highly porous anode films on Ni substrates. The films were obtained by in situ deposition of the particles without any binder or excess carbon black avoiding any secondary treatment. Compared to similarly prepared uncoated SnO2 nanoparticles as well as conventionally prepared powder samples the capacity loss of the in situ deposited nanocomposite films is significantly reduced. Thus, this newly developed anode material combined with in situ film formation is a promising approach for high capacity anodes in Li-ion batteries.
In situ SEM studies
A novel experimental concept was developed for the in situ SEM investigating of electrodes for lithium-ion batteries. It uses an ionic liquid based electrolyte which allows the operation of a special battery under vacuum. With this it is possible to observe a battery inside an SEM under high resolution. This concept could be possibly adapted to other investigation methods such as transmission electron microscopy or photoelectron spectroscopy. Using this method, experiments on SnO2 were performed that reveal some of the active mechanisms in this material and show that the electrochemical behavior of this material strongly depends on particle size.
References
|
[1] |
S. Indris, M. Scheuermann, S. Becker, V. Šepelák, J. Suffner, C. Feldmann, A. Ulrich, H. Hahn, Local Structural Disorder and Relaxation in SnO2 Nanostructures Studied by 119Sn MAS NMR and 119Sn Moessbauer Spectroscopy, J. Phys. Chem. C 115, 6433 (2011) |
|
[2] |
V. Šepelák, K. D. Becker, I. Bergmann, S. Suzuki, S. Indris, A. Feldhoff, P. Heitjans, A One-Step Mechanochemical Route to Core–Shell Ca2SnO4 Nanoparticles Followed by 119Sn MAS NMR and 119Sn Mössbauer Spectroscopy, Chem. Mater. 21, 2518 (2009) |
|
[3] |
S. M. Becker, M. Scheuermann, V. Sepelak, A. Eichhöfer, D. Chen, R. Mönig, A. S. Ulrich, H. Hahn, S. Indris, Electrochemical insertion of lithium in mechanochemically synthesized Zn2SnO4, Phys. Chem. Chem. Phys. 13, 19624 (2011) |
|
[4] |
R. Ochs, D. V. Szabó, S. Schlabach, S. Becker, S. Indris, Development of Nanocomposites for Anode Materials in Li-Ion Batteries, Phys. Status Solidi A 208, 471 (2011) |
|
[5] |
D. Chen, S. Indris, M. Schulz, B. Gamer, R. Mönig, In situ SEM on Lithium-Ion Battery Electrodes Using an Ionic Liquid, J. Power Sources 196, 6382 (2011) |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF