E2.3: Behavior of Cells in 2D and 3D Micro- and Nanostructured Substrates
Subproject Leader: Martin Bastmeyer
Contributing Scientists:
Present: Tatjana Autenrieth, Dr. Alexandra Greiner, Maria Jäckel, Benjamin Richter
Past: Zhongxiang Jiang, Franziska Klein, Thomas Striebel
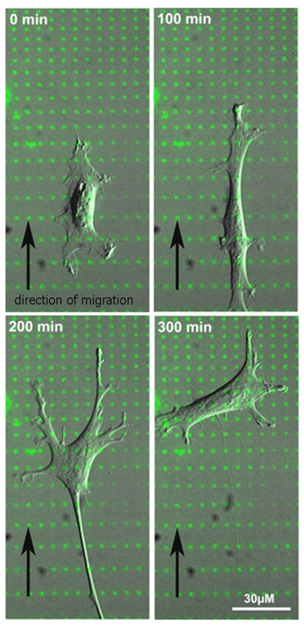
Still frames of a time-lapse movie (DIC image of a cell, fibronectin is shown in green).
3D reconstruction of cardiomyocytes labeled for f-actin (green) and α-actinin (red) growing in an Ormocomp scaffold consisting of posts connected by flexible beams.
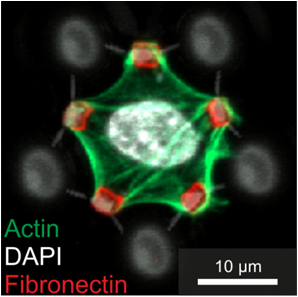
Cells cultured in anti-adhesive 3D scaffolds (grey) containing bio-functionalized areas (red) specifically adhere to the fibronectin-coated dots.
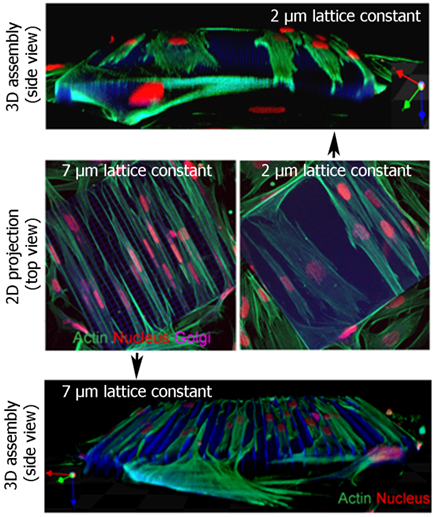
Elastic 3D lattice structures with two mesh sizes (2 µm and 7 µm) are used to culture fibroblasts. Reconstructions of a confocal image stack (actin = green, nucleus = red, Golgi = purple).
During recent years it has become clear that cells are not only influenced by biochemical cues but also by physical aspects like stiffness and geometry of the extracellular environment. These additional factors are simultaneously sensed on different length scales, ranging from nanoscale assembly processes at single sites of cell adhesion to microscale organization of the cytoskeleton. These physical parameters have a major impact on cell fate and function, with dramatic consequences for tissue function. Most of our current knowledge on cell behavior and differentiation is derived primarily from studies on rigid and planar two-dimensional (2D) tissue culture substrates that are homogeneously coated with biomolecules. There is an increasing demand for in vitro models that capture more of the relevant complexity present in three-dimensional (3D) tissue scaffolds. In our projects we use various techniques to functionalize surfaces with biomolecules in a controlled density and a defined geometry. In addition, we have developed new methods to manufacture complex tailored 3D microstructures as a growth substrate for various cell types to study the influence of physical aspects on cell behavior in a manageable 3D environment.
Polarization and Haptotaxis on Micropatterned Fibronectin-Gradients in Primary Fibroblasts
Cell polarization and migration are essential for the function of multicellular organisms. Directed cell movement induced by gradients of soluble signaling molecules is a well-studied phenomenon and referred to as chemotaxis. In contrast, much less is known about cell migration in substrate-bound adhesive protein gradients (haptotaxis).
We use microcontact printing (µCP) to produce discontinuous adhesive fibronectin gradients [Philipsborn et al., 2007]. Primary chicken fibroblasts recognize this pattern and migrate uphill the adhesive gradient. This system offers the possibility to answer basic biological questions: How do cells recognize and read out adhesive gradients? What is the temporal sequence of intracellular organelle reorientation and remodelling of the cytoskeleton during cell polarization and haptotaxis? What are the intracellular signaling mechanisms involved in this process?
Cell Culture in Tailored 3D Microstructure Scaffolds
Our current knowledge on cell behavior and differentiation is primarily derived from studies on rigid and planar two-dimensional (2D) tissue culture substrates. Cell behavior and differentiation are, however, not only influenced by biochemical cues but also by physical properties like adhesive geometry, topography, and stiffness of the three-dimensional (3D) extracellular environment. Therefore, in vitro model systems that capture more of the complexity present in 3D tissue scaffolds are highly desirable for single cell analysis. We realize (in cooperation with the group of Prof. Dr. Martin Wegener, subproject A1.4) 3D microstructure scaffolds by means of Direct Laser Writing (DWL) into biocompatible photoresists (Nanoscribe system). To incorporate in vivo elasticity we also focus on the fabrication of flexible 3D structures using Ormocer® as photoresist. These elastic 3D scaffolds can be rhythmically deformed by single beating cardiomyocytes and are used for single cell force measurements. Calibration with an atomic force microscope in cooperation with the CFN Research Group of Dr. Clemens Franz indicates that cellular forces down to 10-20 nN are detectable with this setup [Klein et al., 2010].
By sequential DLW of two different photoresists, composite polymer scaffolds with distinct mechanical and protein-binding properties can be fabricated. Cells cultured in these scaffolds specifically form cell-adhesion sites with the bio-functionalized parts. Thus, cell adhesion and consequently cell shape can be fully controlled in 3D for the first time. [Klein et al., 2011].
In collaboration with the group of Prof. Dr. Ulrich Schwarz (University Heidelberg) we develop theoretical modeling approaches and computer simulations to understand the underlying biophysical mechanisms of cell shape and force generation in 3D scaffolds [Bischofs et al., 2008].
Mechanical Properties of Cells and Cell Nuclei During Invasion into 3D Microstructures
Cell migration plays an important role not only during the development of an organ, wound healing, and immune response, but also in pathological processes such as tumor metastasis. In this project, we systematically investigate cancer cell lines invading 3D scaffolds with different lattice constants, substrate stiffness, and surface coverage. Since nuclear size and nuclear deformability are limiting factors for cell invasion, we modulate the mechanical properties of the nucleus by siRNA-mediated lamin-knockdown.
References
|
[1] |
F. Klein, B. Richter, T. Striebel, C.M. Franz, G. von Freyman, M. Wegener and M. Bastmeyer, Two-Component Polymer Scaffolds for Controlled Three-Dimensional Cell Culture, Adv. Mater. 23, 1341 (2011) |
|
[2] |
F. Klein, T. Striebel, J. Fischer, Z. Jiang, C.M. Franz, G. von Freyman, M. Wegener and M. Bastmeyer, Elastic fully three-dimensional microstructure scaffolds for cell force measurements, Advanced Mater. 22, 868 (2010) |
|
[3] |
I.B. Bischofs, F. Klein, D. Lehnert, M. Bastmeyer, and U.S. Schwarz, Filamentous Network Mechanics and Active Contractility Determine Cell and Tissue Shape, Biophys. J. 95, 3488 (2008) |
|
[4] |
A.C.von Philipsborn, S. Lang, Z. Jiang , F. Bonhoeffer, and M. Bastmeyer, Substrate-Bound Protein Gradients for Cell Culture Fabricated by Microfluidic Networks and Microcontact Printing, Science STKE: DOI: 10.1126/stke. 4142007pl62007 (2007) |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF