F2.2: Reaction Kinetics of Nanostuctured SOFC Cathodes
Subproject Leader: Ellen Ivers-Tiffée
Institut für Werkstoffe der Elektrotechnik (IWE), KIT
Contributing Scientists: Levin Dieterle, Jan Hayd, Jochen Joos, Christoph Peters, Bernd Rüger, André Weber
Why Going Nano with Fuel Cells?
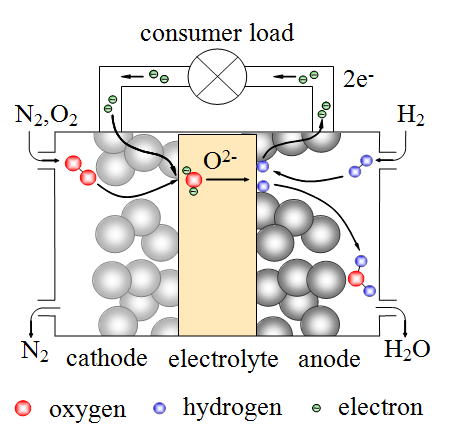
Fuel cells are electrochemical devices which can directly convert chemical energy into electrical energy at a high level of efficiency. Especially solid oxide fuel cells (SOFC) are of particular interest, as they can be operated not only by hydrogen, but also by hydrocarbons, without the need for noble-metal catalysts. To overcome the low ion conductivity of the applied ceramic electrolyte materials, this type of fuel cell is typically operated at temperatures of 750 °C and higher.
The current goal of fuel cell development however is to bring operation temperatures down to 500 °C and lower. This will on the one hand facilitate thermal insulation, especially for small power generators for portable devices, and on the other hand shorten startup times, which are necessary for heating the system.
Nanoscaled dimensions of the functional layers of a fuel cell are the key for low temperature operation. As the thermally activated ion conductivity of the electrolyte drastically increases with decreasing temperature, nanoscaled electrolytes can significantly lower the associated losses by the reduction of the diffusion length [1]. But not only transport phenomena are thermally activated, also the electrode reactions are. Recent electrochemical studies on nanoscaled anodes [2] and cathodes [3, 4] revealed a significant improvement in terms of a reduction of the electrochemical losses. Especially nanoscaled cathodes achieved record breaking performances at temperatures of 400 – 600 °C [4]. The huge performance increase is to a large extent the result of the very high inner surface area generated by nanoscaled particles and pores.
How to Make Nanoscaled Cathodes?
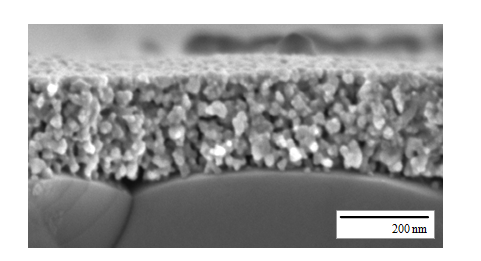
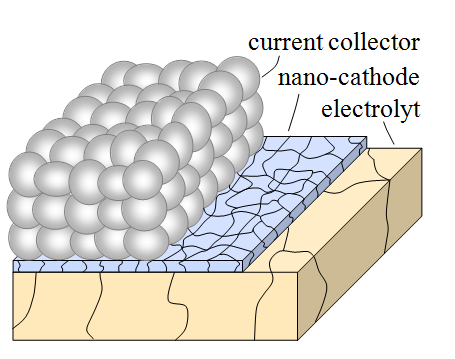
In cooperation with the Fraunhofer Institut für Silicatforschung (ISC) in Würzburg, the deposition of nanoscaled cathode material by a sol-gel process was developed. This process based on metal organics (metal organic deposition, MOD) is already well established for coating of window glass with functional surface layers. By thorough adjustments of the processing and the sol chemistry the method was adapted for the deposition of nanoscaled thin film cathodes. In doing so, we succeeded to optimize the process for the perovskite material La0.6Sr0.4CoO3-δ, resulting in a 50-fold reduction of the polarisation losses compared to µm-scaled cathodes [4]! The process is also ideal for tailoring microstructural properties such as grain size and studying their effect on the cathodic reaction kinetics.
Is it Really Only a Matter of Size?
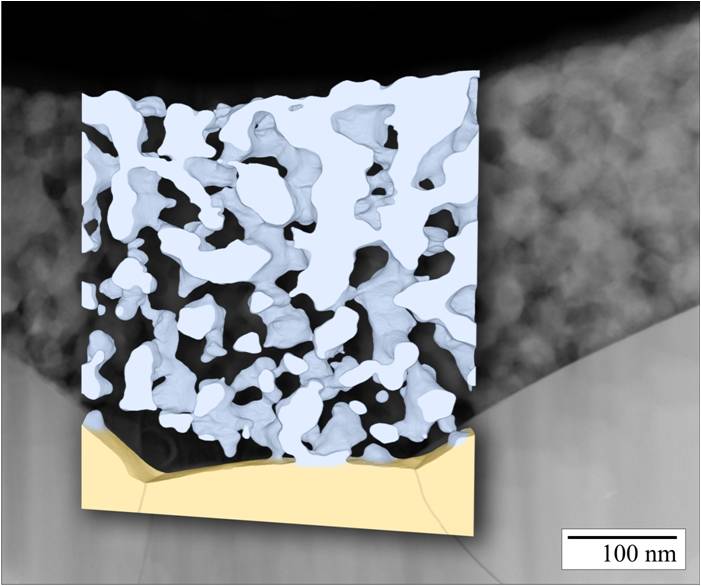
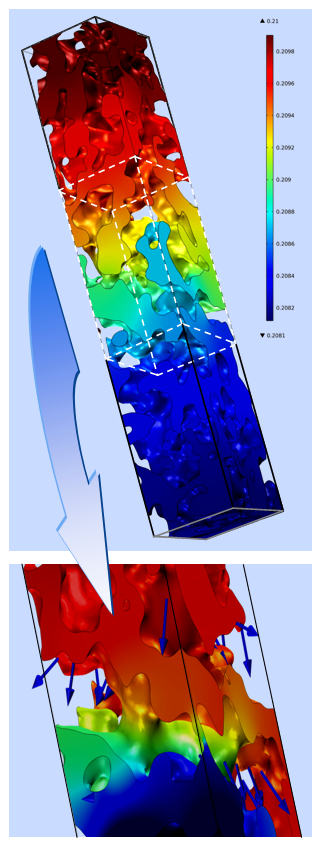
The project is in close cooperation with Dagmar Gerthsen’s group, who performs microstructural and analytical characterizations by means of transmission-electron microscopy. This includes the analysis of the microstructure by conventional transmission electron microscopy (TEM) and high-resolution TEM (HRTEM). A quantum leap in microstructure reconstruction of nanoscaled SOFC-cathodes was achieved by scanning transmission electron microscopy (STEM)-tomography in combination with a specialized image processing, enabling a 3D reconstruction of a nanoscaled thin film cathode with a high and a so far unmatched level of detailing [5]. With this precise microstructural data it was possible to calculate the performance of the nanoscaled cathode structures by 3D FEM simulations and 1D model calculations. However, the size effect could not fully explain the extraordinary results. Additional investigations on the chemical composition and the phase of the cathode material by selected area electron diffraction (SAED), energy-dispersive X-ray spectroscopy (EDXS), energy-filtered TEM (EFTEM) and electron-energy-loss spectroscopy (EELS) suggested that also chemical effects, such as the segregation of nanoparticulate binary oxides, might play a significant role. Detailed analyses of the reaction kinetics of MOD derived nanoscaled thin film cathodes will give further insight into to this complex matter.
References
|
[1] |
C. Peters, A. Weber, B. Butz, D. Gerthsen and E. Ivers-Tiffée, J. Am. Ceram. Soc. 92, 2017 (2009) |
|
[2] |
D. Klotz, B. Butz, A. Leonide, J. Hayd, D. Gerthsen and E. Ivers-Tiffée, J. Electrochem. Soc. 158, B587 (2011) |
|
[3] |
C. Peters, A. Weber und E. Ivers-Tiffée, J. Electrochem. Soc. 155 (7), B730 (2008) |
|
[4] |
J. Hayd, L. Dieterle, U. Guntow, D. Gerthsen, and E. Ivers-Tiffée, J. Power Sources 196, 7263 (2011) |
|
[5] |
L. Dieterle, P. Bockstaller, D. Gerthsen, J. Hayd, E. Ivers Tiffée, and U. Guntow, Adv. Enegy Mater. 1, 249 (2011) |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF