E1.5: Use of Nanoparticles to Study and Manipulate the Polarity of Plant Cells
Subproject Leader: Peter Nick
Botanisches Institut, KIT
Contributing Scientists:
Present: Jan Klotz, Kai Eggenberger
Past: Steffen Durst, Aleksandra Zimmermann, Gregor Rottwinkel
What are We Aiming For?
We use the novel materials and tools from the nanosciences to manipulate plant cells. Plants are located at the start of the food chain. To understand the environmental impact of nanomaterials, one therefore needs to analyze their effect on plant cells. In addition, we want to use nanomaterials to understand the totipotency of plant cells (i.e. the ability to generate an entire organism like stem cells do in animals). We have shown previously that this ability is linked to actin.
Actin is the building block of our muscles, Plants possiss actin as well, although theiy cannot move. We think that actin has developed into a tool, by which plants can sense environmental direction. We have deloped a couple of tools by which we can bundle or relax actin filaments through genetic engineering. However, this approach only allows global manipulation of plant cells. To probe directionality, we need new approaches to manipulate actin filaments in different cells or even in different regions of a single cell. Herefore, we use the tools provided by the nanosciences.
Why we Need Chemical Engineering
Genetic engineering, the manipulation of life by reprogramming the DNA has developed into a central tool of current biology. However, many organisms are not easily amenable to genetic transformation. Moreover, at the cellular level biology is often controlled by spatial patterns of molecules. It is not only important that a certain molecule is generated, it is important, where in the cell this happens. Chemical engineering is the approach to steer cellular events directly (i.e. not via altering the DNA) through tailored molecules. It can be administered also in organisms that are recalcitrant to transformation and also allows for spatial patterning. It thus complements the tool box of current biology.
What are the Challenges for Chemical Engineering?
The tools of chemical engineering should be as precise as possible. The price for precision is size – these structures (such as nanoparticles, peptides, or complex organic molecules) have to pass the cell membrane, in plants, they have, in addition, to pass a cellulosic cell wall. Once they are in the cell, they have to be addressed to the correct cellular target. In the long term, the activity of these tools should become controlable in space and time by a switch such as light. Our project addresses these challenges for plant cells.
How to Get In? Trojan Peptoids as Vehicles
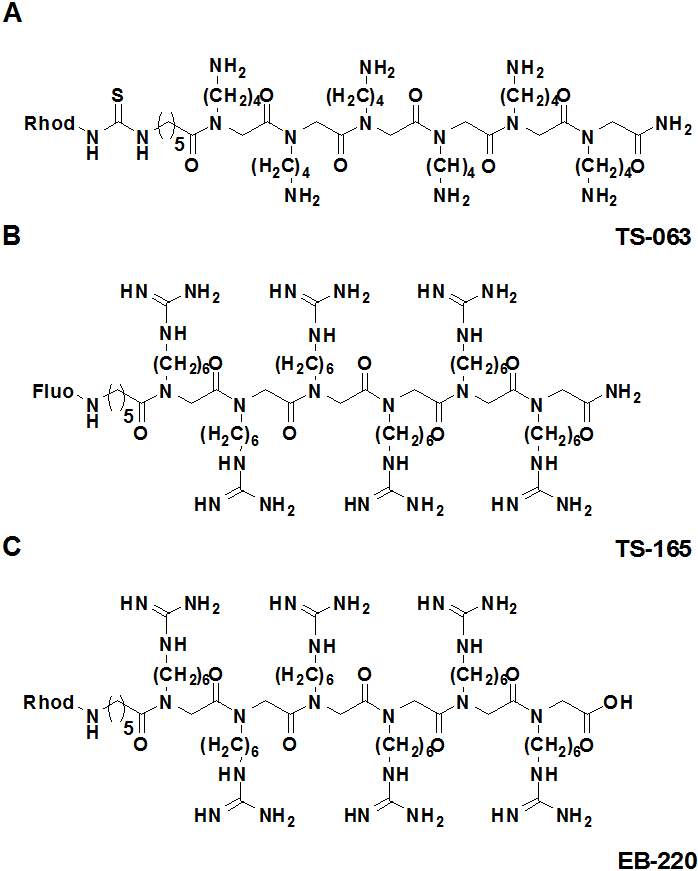
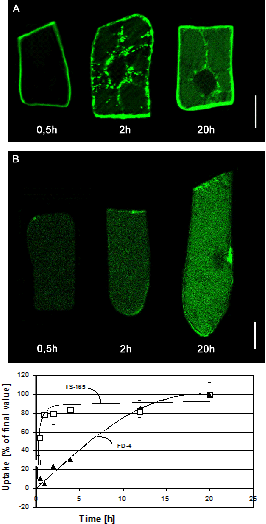
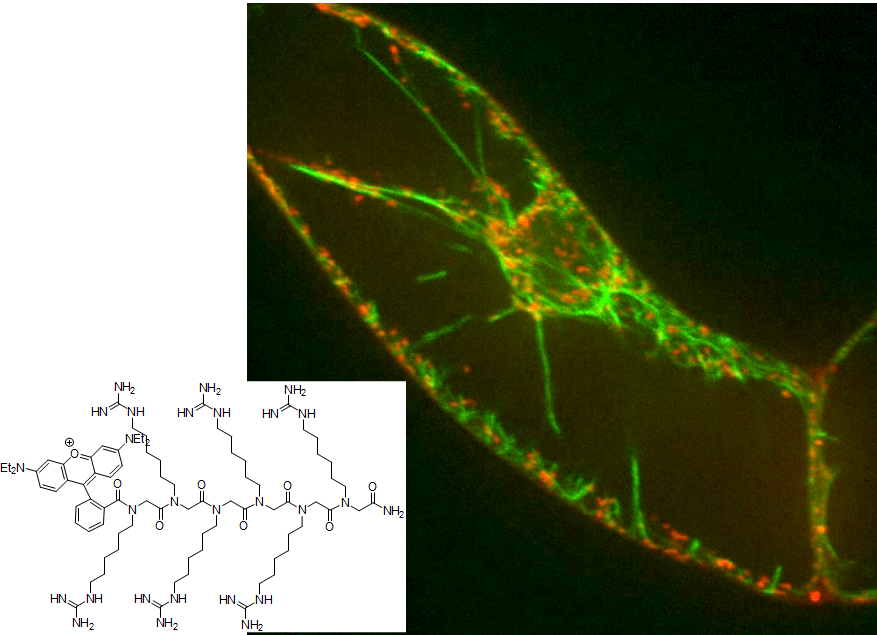
In cooperation with the group of Stefan Bräse (project E1.1) we investigate so called Trojan Peptoids, designed imitations of peptides that can permeate by an unknown mechanism through intact membranes. In contrast to peptides, the side groups attach to the nitrogen atom, such that peptoids cannot be attacked by degrading enzymes. We could show that fluorescently labelled poly-guanidine-peptoids enter plant cells without affecting their viability (Fig. 1). The uptake is independent from endocytosis, but requires an intact cytoskeleton. A third-generation peptoid can be specifically targeted to plant mitochondria. This allowed to show for the first time to follow the movement of plant mitochondria along actin filaments by high resolution 4D confocal microscopy (Fig. 2). The movement data allowed to derive quantitative statements and demonstrated that the movement is driven by the assembly of actin monomers at the growing end of the filament.
How to Reach the Correct Target? Cell-Permeating Peptides with “Address”
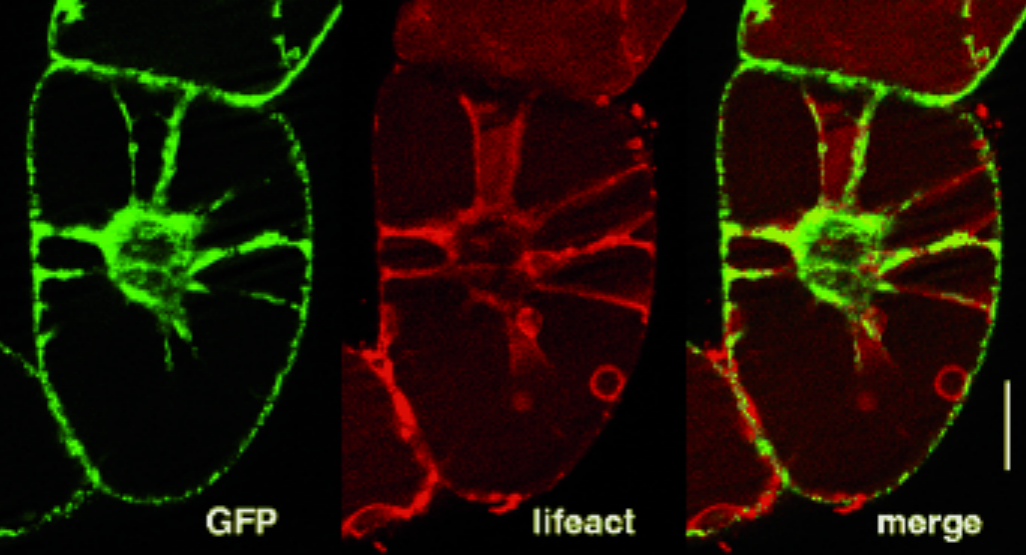
In parallel to the peptoids, we have developed, in cooperation with the group of Anne Ulrich (E1.2) a strategy based on positively charged peptides that can pass the membrane, again by unknown mechanisms. The synthesis of peptides with defined sequence is a standard method and allows to generate structures that can interact specifically with target proteins. This allows to address the second challenge of chemical engineering. In a proof-of-principle approach we used the artificial peptide BP100 as vehicle, and linked an actin-binding peptide motif (Lifeact) from yeast actin-binding proteins and a fluorescent marker. Using this construct, we were able, for the first time, to visualize actin in living plant cells without the need for genetic transformation (Fig. 3). This paves the way to alter the spatial pattern of actin (and thus the directionality of the cell) specifically, for instance by microfluidics.
Vision: Chemical Engineering of Immunity and Cellular Self Organisation
We want to extend the molecular transporter system by introducing functional cargoes for chemical engineering of plant cells As short-term goal we want to introduce molecular transporters conveying designed peptides tailored to interfere with protein-protein interactions. Our target is actin-depolymerizing factor 2 (ADF2), a central switch in a self-regulatory network that controls the directionality of plant cells. As long-term application we want to manipulate the immunity of plants against pathogens by chemical engineering. Plant immunity is of high economic relevance – it is estimated that about a third of crop harvests worldwide are destroyed by pathogens. By introducing antimicrobial peptides (such as the chitin-binding peptide from Ginkgo biloba) through a CPP-based transporter system into plant cells it should be possible to engineer immunity in plants that are recalcitrant to genetic engineering.
References
|
[1] |
N. Kusaka, J. Maisch, P. Nick, K.I. Hayashi, and H. Nozaki. ChemBioChem 10, 2195-2202 (2009) (a research highlight in Nature Chemical Biology dedicated to this article) |
|
[2] |
K. Eggenberger, T. Schröder, E. Birtalan, S. Bräse, and P. Nick. ChemBioChem 10, 2504-2512 (2009) |
|
[3] |
K. Eggenberger, N. Frey, B. Zienicke, J. Siebenbrock, T. Schunck, R. Fischer, S. Bräse, E. Birtalan, T. Nann, and P. Nick. Adv. Biomat. 12, 406-412 (2010) |
|
[4] |
Eggenberger, C. Mink, P. Wadhwani, A.S. Ulrich, and P. Nick. ChemBioChem 12, 132-137 (2011) |
|
[5] |
E. Birthalan, K. Eggenberger, P. Nick, and S. Bräse. PloS One, in press |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF
.png)