F3.2: Nanoscale Li-Alloys for Thermoelectrics and Lithium Batteries
Subproject Leader: Claus Feldmann
Institut für Anorganische Chemie, KIT
Contributing Scientists:
Present: Silke Wolf
Why new materials for high-power batteries?
Future energy saving and energy storage will require a reduction of thermal loss processes, an increased overall efficiency as well as efficient storage devices. To this end, high-power batteries become more and more important, aiming at industrial manufacturing, house-hold application as well as individual transport. Right now materials are mainly focused on LiCn/LixMO2 (1<x<2; M: Mn, Fe, Co, Ni) in the case of lithium-ion batteries [1]. To further improve the performance of high-power batteries, especially of lithium-ion batteries, novel materials are however crucially requested. To this regard, novel materials and nanomaterials as potential cathodes and anodes in high-power batteries are addressed in subproject F3.2: Materials for Lithium Batteries.
Less-nobel metal nanoparticles
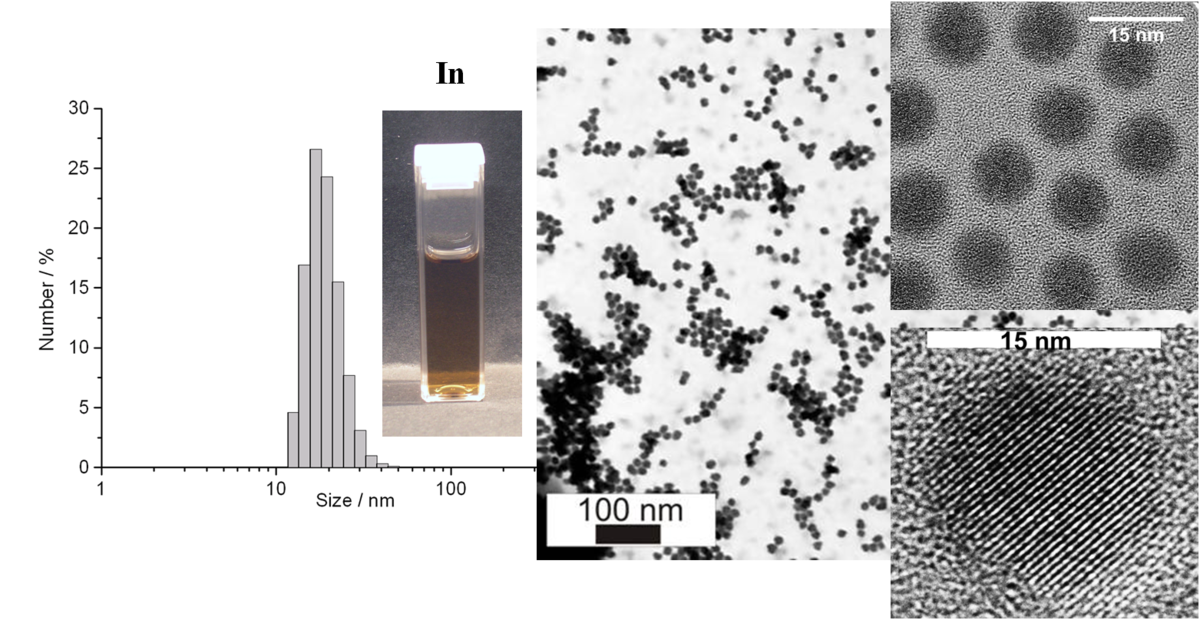
Less-noble metals have been frequently discussed as precursors for lithium-containing alloys which then can serve as anode materials. On the nanoscale, less-noble metals have been barely investigated due to severe restrictions regarding preparation and sensitivity. With this CFN-subproject, the synthesis of uniform In0, Bi0 and Cu0 nanoparticles has been established [2,3]. Manufacturing of thin-film electrodes and Li-containing alloys as well as a fundamental study related to quantum size effects have been performed.
Halogen-rich compounds
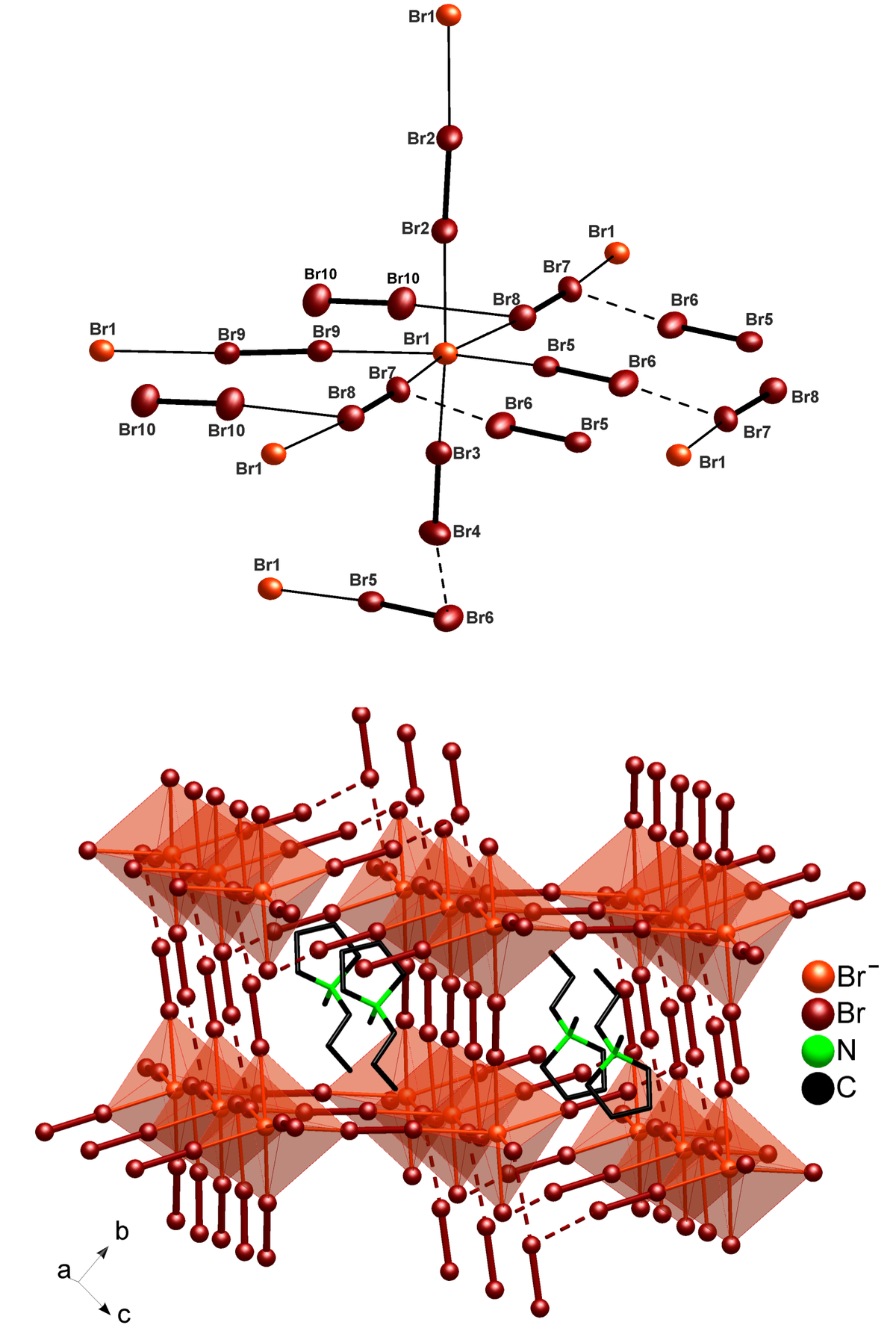
Polybromides have been already discussed in view of high-power batteries such as zinc-bromine and lithium-bromine batteries. By synthesis in ionic liquids, we could prepare four novel polybromides [(Ph)3PBr][Br7], [(Bz)(Ph)3P]2[Br8], [(n-Bu)3MeN]2[Br20] and [C4MPyr]2[Br20] [4]. Hereof, [(n-Bu)3MeN]2[Br20] and [C4MPyr]2[Br20] represent infinite two- and three-dimensional networks that contain the highest amount of dibromine as well as the highest amount of elemental halogen ever observed. Based on its surprisingly high stability, especially [C4MPyr]2[Br20] can be interesting in view of save storage and handling of the reactive bromine as well as for future high-power batteries.
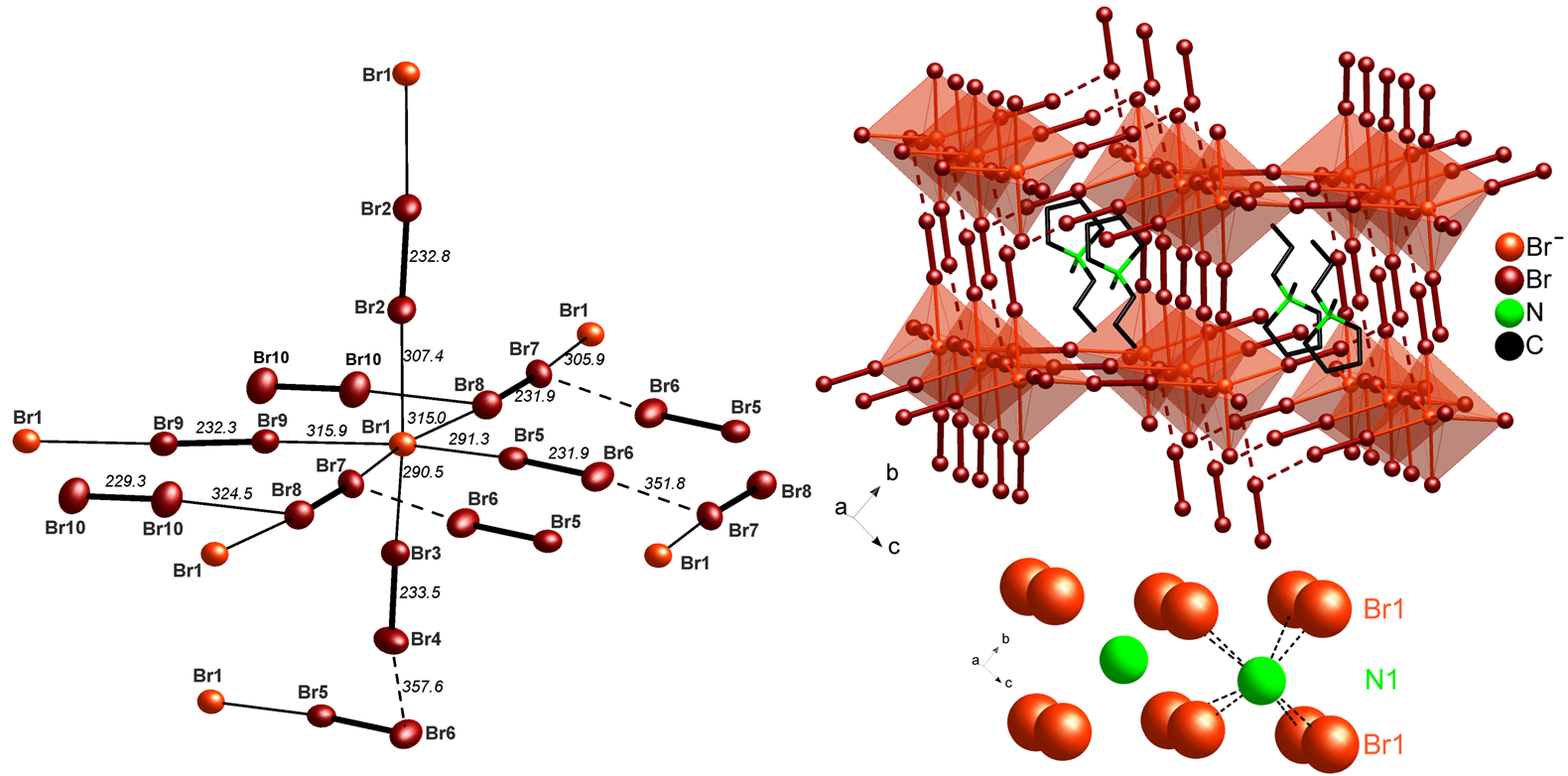
![]() [(Br–)2(Br2)4(2Br2)2(Br2)] network in [C4MPyr]2[Br20] established by distorted corner-sharing [(Br–)(Br2)4(2Br2)2]– octahedra (foreshadowed in light red) with the central bromide anion (Br1) as the network node that is interlinked by dibromine molecules (dark red with bold line). The [C4MPyr]+ cation serves as a templating agent in between of the polybromide 3D-network. Reckoning the central bromide anion as well as nitrogen as the center of the cation only, visualizes the correlation with a distorted CsCl-type structure (bottom) [4].
[(Br–)2(Br2)4(2Br2)2(Br2)] network in [C4MPyr]2[Br20] established by distorted corner-sharing [(Br–)(Br2)4(2Br2)2]– octahedra (foreshadowed in light red) with the central bromide anion (Br1) as the network node that is interlinked by dibromine molecules (dark red with bold line). The [C4MPyr]+ cation serves as a templating agent in between of the polybromide 3D-network. Reckoning the central bromide anion as well as nitrogen as the center of the cation only, visualizes the correlation with a distorted CsCl-type structure (bottom) [4].
References
| [1] | M. Armand, J. M. Tarascon, Nature 2008, 451, 652. |
| [2] | E. Hammarberg and C. Feldmann, In0 Nanoparticle Synthesis assisted by Phase-transfer Reaction, Chem. Mater. 2009, 21, 771 |
| [3] | S. Wolf and C. Feldmann, Cu2X(OH)3 (X = Cl, NO3): Synthesis of Nanoparticles and Its Application for Room Temperature Deposition/Printing of Conductive Copper Thin-films, J. Mater. Chem. 2010, 20, 7694. |
| [4] | M. Wolff, J. Meyer, and C. Feldmann, [C4MPyr]2[Br20] − Ionic Liquid based Synthesis of the first three-dimensional Polybromide Network, Angew. Chem. Int. 2011, 50, 4970 |
List of Publications 2006-2011 as PDF
Subproject Report 2006-2010 as PDF